1. Ethane, with the molecular formula C2H6 has
(a) 6 covalent bonds
(b) 7 covalent bonds
(c) 8 covalent bonds
(d) 9 covalent bonds
Answer:
(b) 7 covalent bonds.
2. Butanone is a four-carbon compound with the functional
group
(a)
carboxylic acid
(b) aldehyde
(c) ketone
(d) alcohol
Answer:
(c) Ketone.
3. While cooking, if the bottom of the vessel is getting
blackened on the outside, it means that
(a) the food
is not cooked completely.
(b) the fuel
is not burning completely.
(c) the fuel
is wet.
(d) the fuel
is burning completely.
Answer:
(b) The fuel is not burning
completely.
4. Explain the nature of the covalent bond using the bond
formation in CH3Cl.
Answer:
Covalent bond
is formed by sharing of electrons so that the combining atoms complete their
outermost shell.
Bond formation in CH3Cl:
Electronic configuration of C
is 2,4
Electronic configuration of H is
1
Electronic configuration of Cl
is 2, 8, 7.
Three
hydrogen atoms complete their shells by sharing three electrons (one electron
each) of carbon atom.
Chlorine
completes its outer shell by sharing its one out of seven electrons with one
electron of carbon atom.
5. Draw the electron dot structures for
(a) ethanoic
acid
(b) propanone
(c) H2S
(d) F2.
Answer:
6. What is a homologous series? Explain with an example.
Answer:
Homologous series
is a group of organic compounds having similar structures and similar chemical
properties in which the successive compounds differ by -CH2 group.
E.g. general formula of the homologous series of alkanes is CnH2n+2.
Here, ‘n’ denotes number of carbons.
|
Homologous series of alkanes |
||
|
Value of n |
Molecular formula |
Name of compound |
|
1 |
CH4 |
Methane |
|
2 |
C2H6 |
Ethane |
|
3 |
C3H8 |
Propane |
|
4 |
C4H10 |
Butane |
|
5 |
C5H12 |
Pentane |
7. How can ethanol and ethanoic acid be differentiated on the
basis of their physical and chemical properties?
Answer:
Differences on the basis of
physical properties:
|
Property |
Ethanol |
Ethanoic acid |
|
Odour |
Sweet smell |
Pungent vinegar-like smell |
|
Melting point |
156 K |
290 K |
|
Boiling point |
351 K |
391 K |
Differences on the
basis of chemical properties:
|
Test |
Ethanol |
Ethanoic acid |
|
Litmus test |
No change in colour. |
Blue litmus solution turns red. |
|
Sodium hydrogen carbonate test |
C2H5OH + NaHCO3 → No reaction. |
CH3COOH + NaHCO3 → CH3COONa
+ H2O + CO2 Brisk effervescence due to evolution of CO2. |
|
Alkaline potassium permanganate test |
On heating, pink colour disappears. |
No change. |
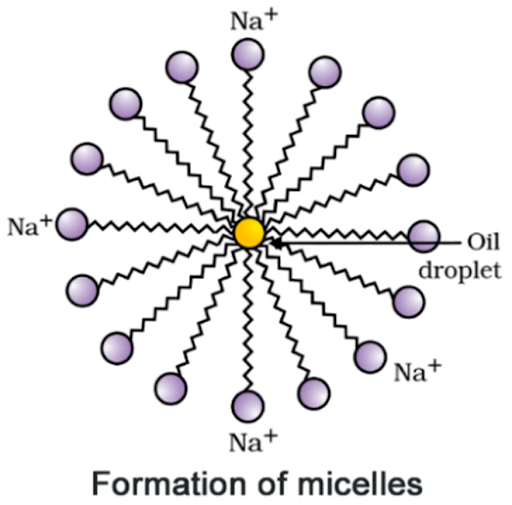
8. Why does micelle formation take place when soap is added
to water? Will a micelle be formed in other solvents such as ethanol also?
Answer:
Micelle
formation takes place because hydrocarbon chains of soap molecules are
hydrophobic and insoluble in water, but the ionic ends of soap molecules are
hydrophilic and soluble in water.
Micelle formation
is not be possible in solvents like ethanol in which sodium salt of fatty acids
do not dissolve.
9. Why are carbon and its compounds used as fuels for most
applications?
Answer:
Carbon and
its compounds give a large amount of heat per unit weight.
10. Explain the formation of scum when hard water is treated
with soap.
Answer:
Hard water
contains salts of calcium and magnesium. On reacting with soap, they form
insoluble precipitate called scum. This reduces the cleansing property of soaps
in hard water.
11. What change will you observe if you test soap with litmus
paper (red and blue)?
Answer:
Red litmus
turns blue because soap is alkaline in nature. Blue litmus remains blue in soap
solution.
12. What is hydrogenation? What is its industrial application?
Answer:
Hydrogenation
is the addition of hydrogen to an unsaturated hydrocarbon to obtain a saturated
hydrocarbon. It takes place in presence of nickel or palladium metals as
catalyst.
Application: It is used to prepare vegetable ghee (or vanaspati ghee)
from vegetable oils.
13. Which of the following hydrocarbons undergo addition reactions?
C2H6, C3H8, C3H6,
C2H2 and CH4
Answer:
C3H6 and
C2H2. Addition reactions occurs only in
unsaturated hydrocarbons.
14. Give a test that can be used to differentiate chemically
between butter and cooking oil.
Answer:
Add bromine
water to some cooking oil and butter taken in each test tubes.
Cooking oil decolourises bromine water showing that it is an
unsaturated compound.
Butter does not decolourise bromine water showing that it is a
saturated compound.
15. Explain the mechanism of the cleaning action of soaps.
Answer:
When a dirty cloth is put in soap water, the hydrocarbon end of the soap molecules in micelle attach to the oil or grease particles on the cloth. The ionic ends of the soap molecules in the micelles remain attached to water. When the dirty cloth is agitated in soap solution, the oily and greasy particles entrapped by micelles get dispersed in water. Thus the cloth gets cleaned.