ACIDS, BASES AND SALTS
MORE ABOUT SALTS
Family of Salts
Salts having same positive or negative radicals belong to a family. E.g. NaCl &
Na2SO4 belong to family of sodium salts. NaCl & KCl
belong to the family of chloride salts.|
Salts
& their Chemical formulae |
Formed from which Acids & bases? |
|
Potassium sulphate (K2SO4) |
H2SO4
& KOH |
|
Sodium sulphate (Na2SO4) |
H2SO4
& NaOH |
|
Calcium sulphate (CaSO4) |
H2SO4
& CaCO3 |
|
Magnesium sulphate (MgSO4) |
H2SO4
& Mg(OH)2 |
|
Copper sulphate (CuSO4) |
H2SO4
& Cu(OH)2 |
|
Sodium chloride (NaCl) |
HCl
& NaOH |
|
Sodium nitrate (NaNO3) |
HNO3
& NaOH |
|
Sodium carbonate (Na2CO3) |
H2CO3
& NaOH |
|
Ammonium chloride (NH4Cl) |
HCl
+ NH4OH |
pH of Salts
Salts of a strong acid & a strong base are neutral (pH = 7).|
Salt |
pH |
Acid used |
Base used |
|
Sodium chloride |
7 |
HCl |
NaOH |
|
Potassium nitrate |
7 |
HNO3 |
KOH |
|
Aluminium chloride |
7 |
HCl |
Al(OH)3 |
|
Zinc sulphate |
7 |
H2SO4 |
Zn(OH)2 |
|
Copper sulphate |
<
7 |
H2SO4 |
Cu(OH)2 |
|
Sodium acetate |
>
7 |
CH3COOH |
NaOH |
|
Sodium carbonate |
>
7 |
H2CO3 |
NaOH |
|
Sodium hydrogen carbonate |
>
7 |
H2CO3 |
NaOH |
Chemicals from Common
Salt
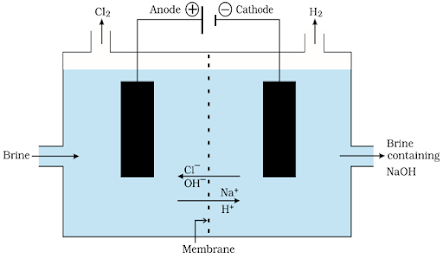
Common salt (sodium chloride, NaCl) is a neutral salt formed by the reaction of HCl & NaOH solution.1. Sodium
hydroxide (NaOH)
2NaCl(aq) + 2H2O(l) → 2NaOH(aq) + Cl2(g) + H2(g)
Important products from the
chlor-alkali process
2. Bleaching
powder (CaOCl2)
Chlorine acts on dry slaked lime to give bleaching powder.
Ca(OH)2 + Cl2 → CaOCl2 + H2O
Actual
composition of CaOCl2 is quite complex.
Uses of Bleaching powder:
- For bleaching cotton & linen in the textile industry.
- For bleaching wood pulp in paper factories.
- For bleaching washed clothes in laundry.
- As an oxidising agent in many chemical industries.
- To make drinking water free from germs.
3. Baking
soda
When it is heated for cooking, the following reaction occurs.
Uses of Baking soda:
- To make tasty crispy pakoras, etc.
- It is added for faster cooking.
- To make baking powder (baking soda + mild edible acid such as tartaric acid). When baking powder is heated or mixed in water, the following reaction occurs:
NaHCO3 + H+ → CO2 + H2O + Sodium
salt of acid
(From any acid)
CO2
causes bread or cake to rise making soft and spongy.
- It is an ingredient in antacids. Being alkaline, it neutralises excess acid (acidity) in stomach.
- Used in soda-acid fire extinguishers.
4. Washing
soda (Na2CO3.10H2O)
NaCl
→ NaHCO3 → Na2CO3 (sodium carbonate).
Recrystallisation
of sodium carbonate → washing soda.
Na2CO3
+ 10H2O
→ Na2CO3.10H2O
Uses of washing soda:
- Used in glass, soap and paper industries.
- To manufacture sodium compounds such as borax.
- Used as a cleaning agent for domestic purposes.
- For removing permanent hardness of water.
Are the Crystals of Salts
really Dry?
Heat few copper sulphate crystals in a dry boiling tube.Plaster
of Paris
CaSO4.
½ H2O +1½ H2O → CaSO4.2H2O
(Plaster of Paris) (Gypsum)
Uses of Plaster of Paris:
- Used as plaster to support fractured bones.
- To make toys and materials for decoration.
- To make surfaces smooth.
Plaster
of Paris gets its name from large gypsum deposits in Montmartre in Paris.